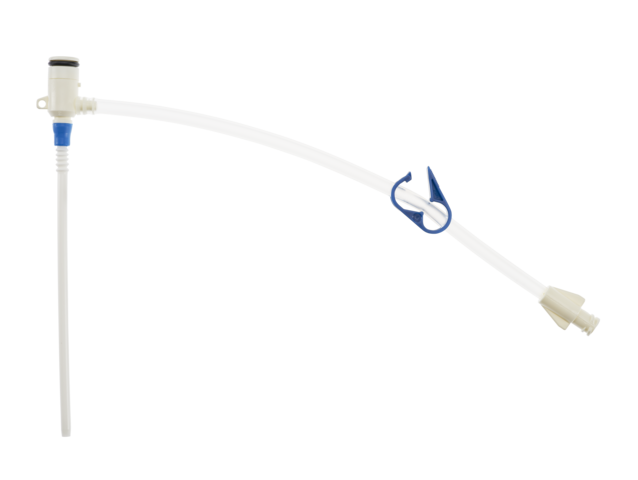
Representative photo(s): actual product specifications may vary from that shown in the photo
PSI for use with 7.5 - 8 Fr. Catheters
PSI KIT: 9 FR X 4-1/8IN (10 CM)
SKU / Article #: NA-09903-CDC
Highlights
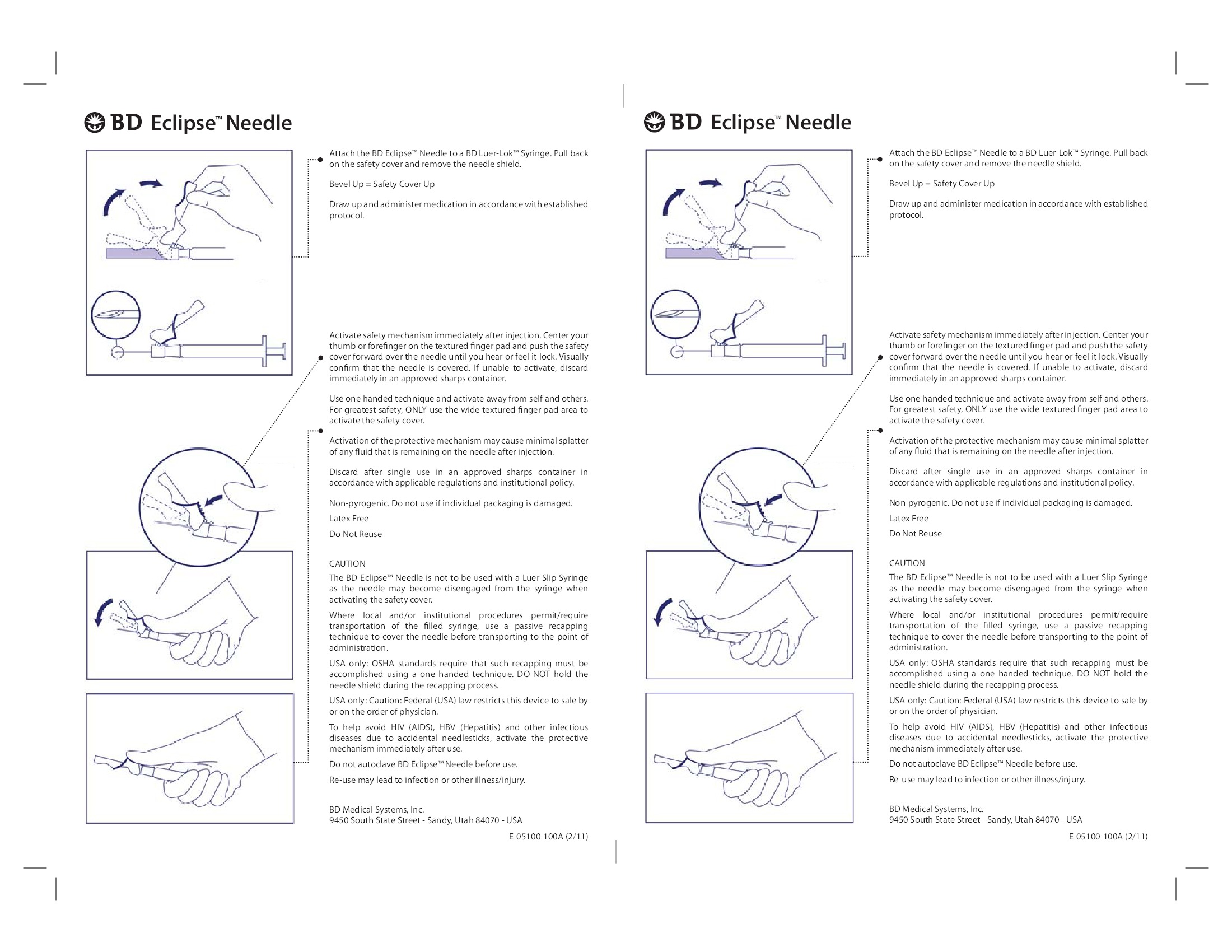
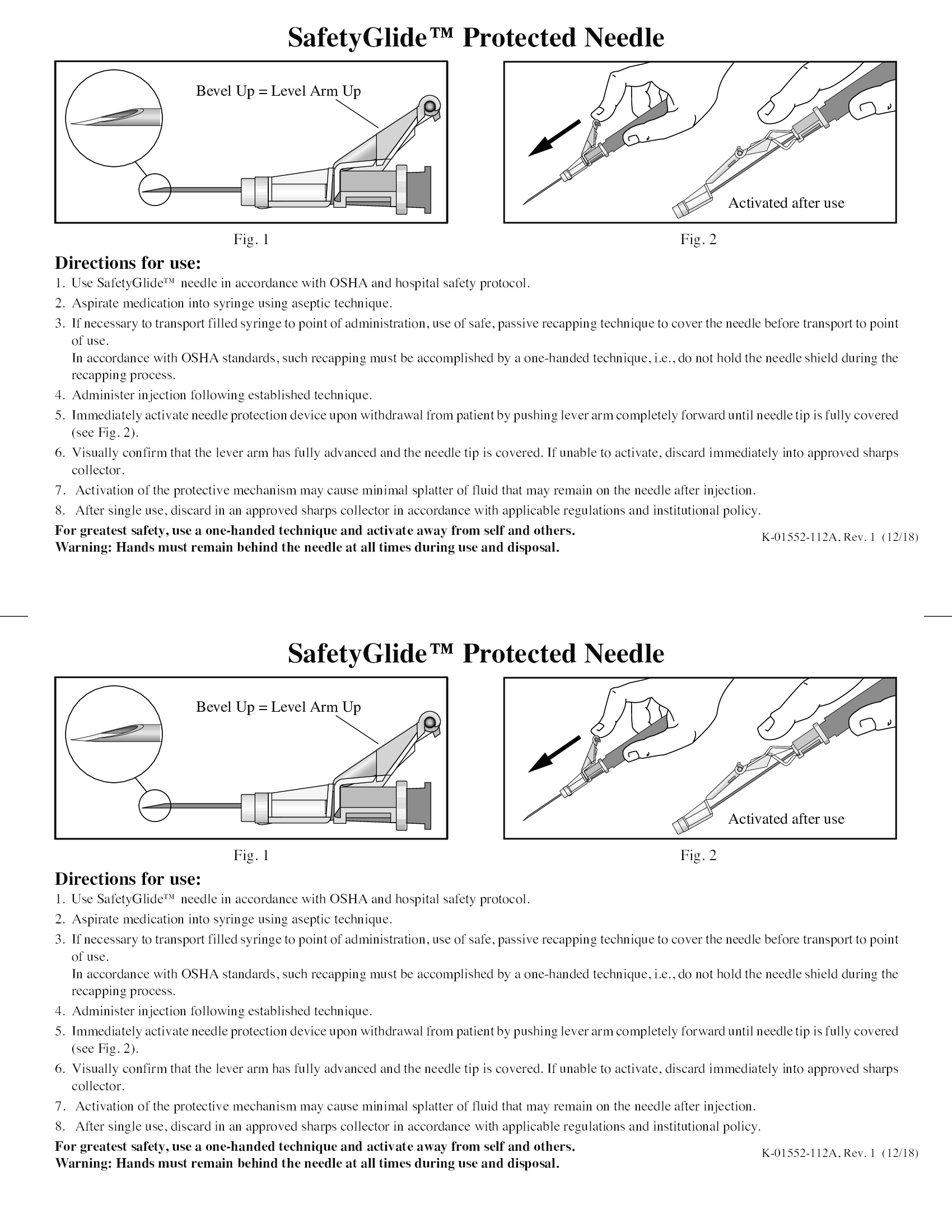
- Sharps Safety
Considerations
- Not made with natural rubber latex.
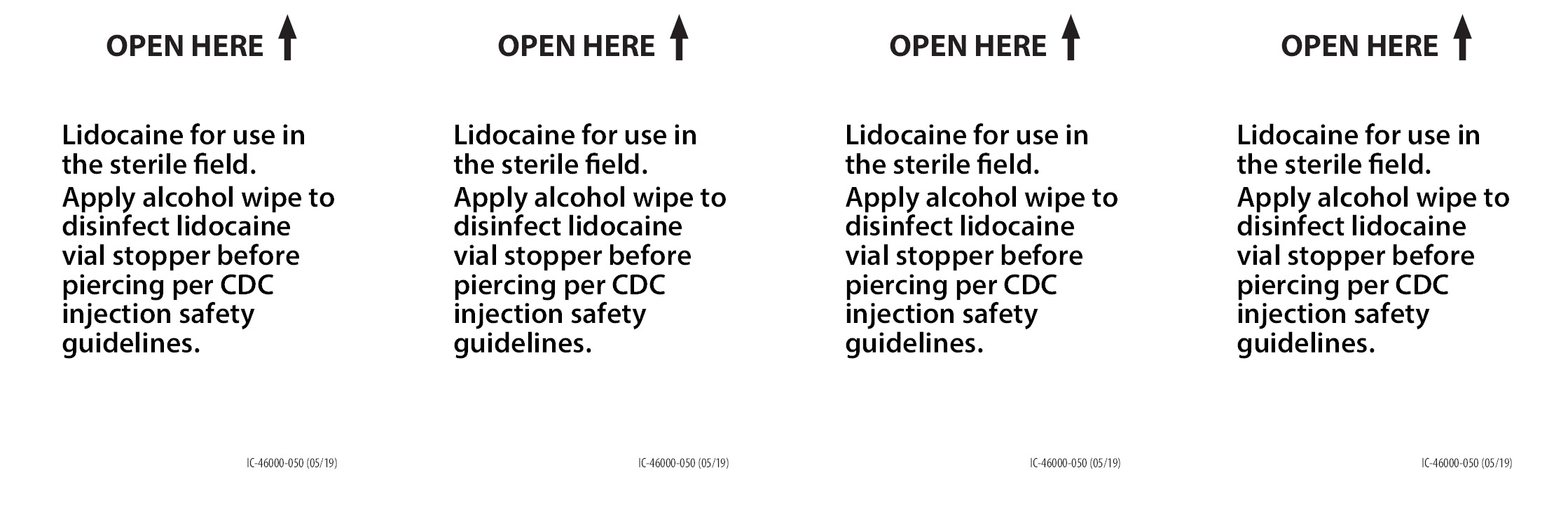
- Includes Lidocaine
- ±Precaution: Contains Phthalate: DEHP
Sales Quantity/Case: 5
1 A trademark of Becton, Dickinson and Company.
2 A registered trademark of CareFusion or one of its subsidiaries.
Documents
Scroll to View More
Scroll to View More
Scroll to View More
The products in this catalog may not be available in all countries. Please contact your local representative.
Indications, contraindications, warnings and instructions for use can be found in the Product Instructions for Use available under the Documents section on this page, where an IFU is available.